ក្រុមហ៊ុនផលិតឧបករណ៍ព្យាបាលពោះវៀនធំ
Colon Hydrotherapy Machine Safety Standards You Need to Know
September 26, 2025










Why ម៉ាស៊ីនព្យាបាលទឹកប្រដាប់ជាតិអ៊ីដ្រូពោព Safety Standards Matter
Safety standards for Colon Hydrotherapy Machines serve multiple critical purposes beyond mere regulatory compliance. These standards ensure consistent water temperature control, prevent backflow contamination, and maintain appropriate pressure levels during treatments. Without proper certification, your equipment may pose serious risks to patients and expose your practice to liability.
The consequences of using non-compliant equipment can be severe:
- Patient injury from improper pressure or temperature
- Cross-contamination between patients
- Electrical hazards from substandard components
- Legal liability and potential practice closure
- Insurance coverage limitations or denials

Need Expert Guidance on Safety Compliance?
Our specialists can help you understand which certifications are mandatory for your region and practice type.
Key International Safety Certifications for Colon Hydrotherapy Machines
Different regions have specific certification requirements for medical devices like Colon Hydrotherapy Machines. Understanding these certifications helps you ensure your equipment meets local regulations and international standards of safety and quality.

| ការបហ្ជាក់ដោយលិខិត | តមបន់ | ការបិបន៍នា | Importance |
| CE Mark | European Union | Confirms compliance with EU health, safety, and environmental protection standards | Mandatory for EU market access |
| FDA Clearance | United States | Verifies safety and effectiveness according to US standards | Required for US market |
| ISO 13485 | International | Quality management system for medical devices | Industry standard for quality |
| IEC 60601-1 | International | Electrical safety standards for medical equipment | Critical for electrical safety |
| EN 1717 | European Union | Backflow prevention in water systems | Essential for preventing contamination |
Technical Safety Standards for Colon Hydrotherapy Machines
Beyond certifications, specific technical standards govern the safety features of Colon Hydrotherapy Machines. These standards address critical aspects like water temperature control, pressure regulation, and contamination prevention.
Water Temperature Control

Safe machines must maintain precise water temperature between 36-38°C and include automatic shutdown if temperature exceeds safe limits.
Pressure Regulation

Compliant devices feature precise pressure control systems with automatic pressure relief valves to prevent patient injury.
Contamination Prevention
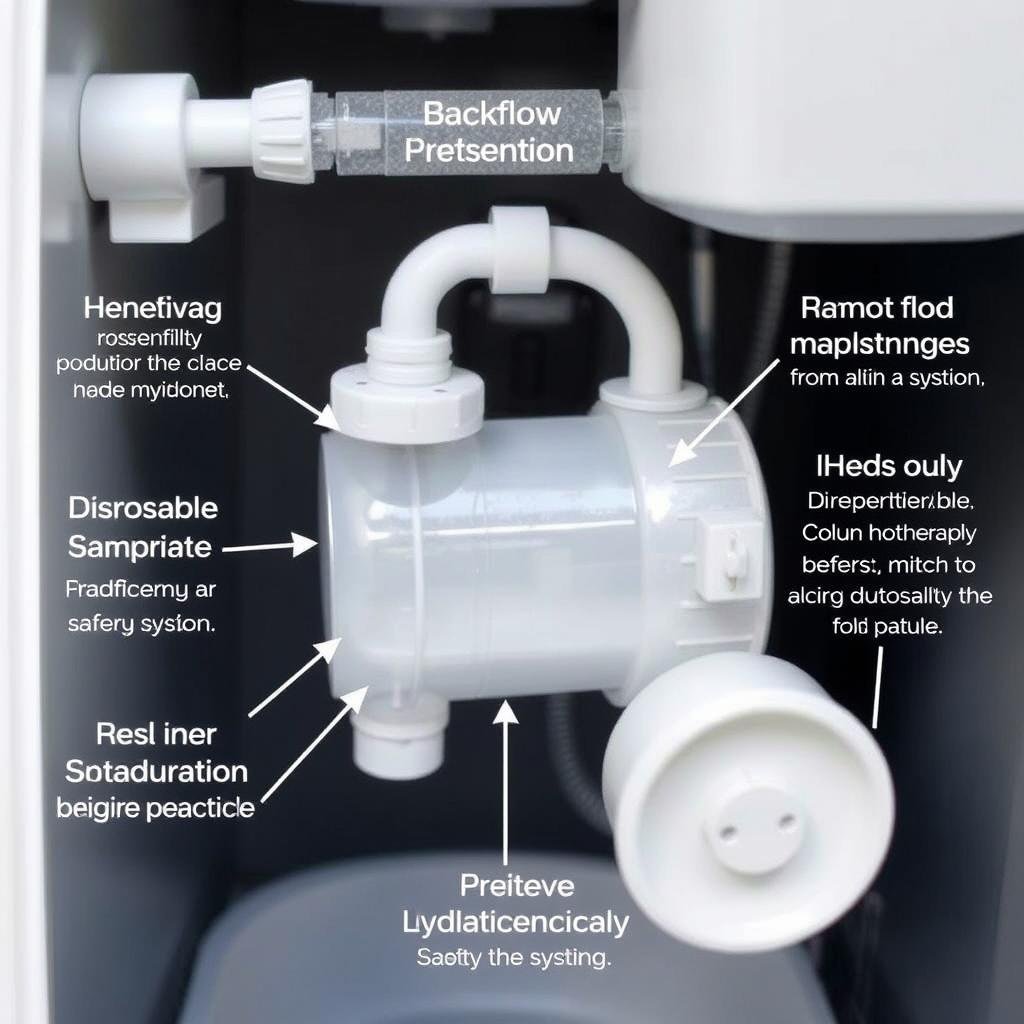
EN 1717 compliant backflow prevention systems and disposable components are essential for preventing cross-contamination.
“The most critical aspect of any ម៉ាស៊ីនព្យាបាលទឹកប្រដាប់ជាតិអ៊ីដ្រូពោព is its safety system. Without proper temperature control, pressure regulation, and contamination prevention, even the most advanced features become irrelevant.”
Closed vs. Open Systems: Safety Considerations
When evaluating Colon Hydrotherapy Machines, understanding the fundamental differences between closed and open systems is crucial for safety considerations.
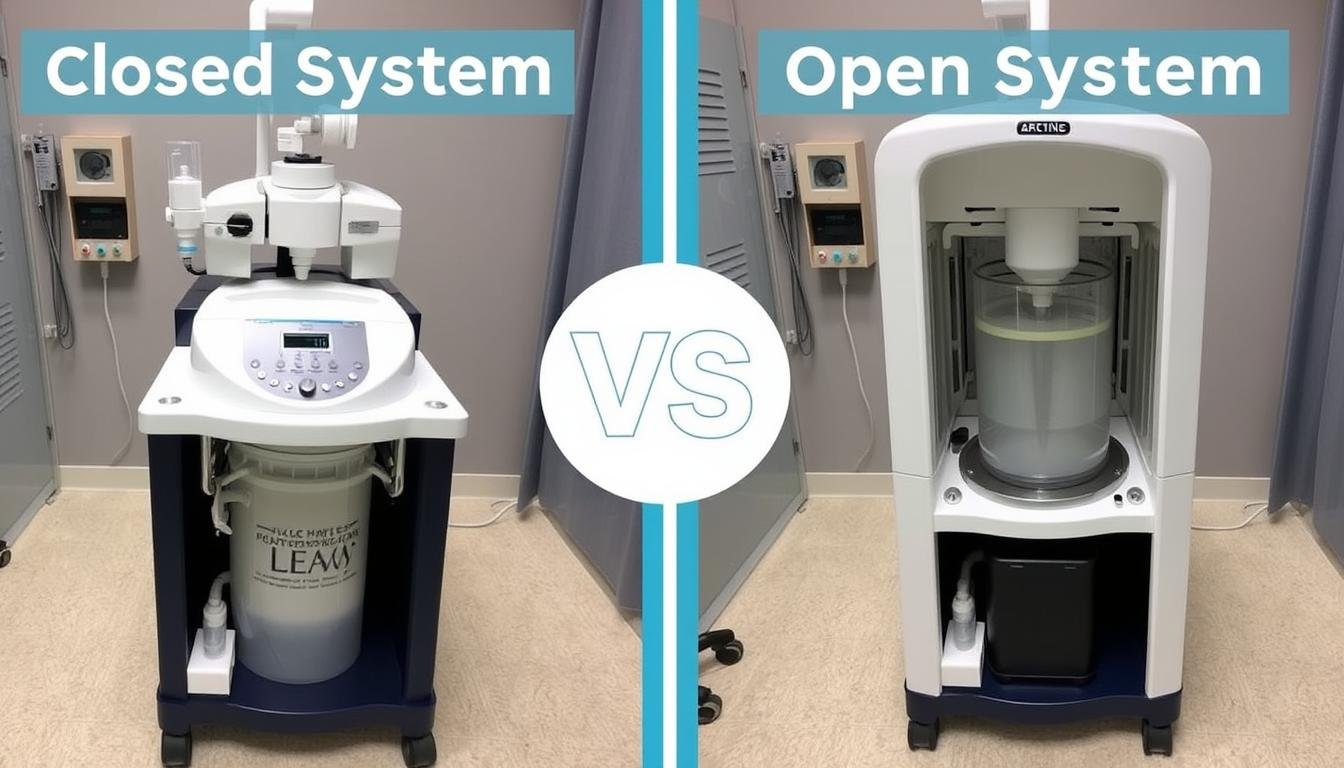
Closed System Safety Features
- No open evacuation and no offensive odours
- Reduced risk of environmental contamination
- Therapist-assisted for effectiveness and safety
- Precise temperature and pressure control
- Self-cleaning capabilities with disinfection systems
Open System Considerations
- Higher risk of environmental contamination
- More challenging to maintain consistent pressure
- May require additional ventilation systems
- Often less expensive but with fewer safety features
- May not meet all regional certification requirements
Safety Tip: Closed systems generally offer superior safety features and are increasingly becoming the standard in professional settings. They provide better infection control and patient dignity while meeting stricter regulatory requirements.
How to Verify Compliance of Your ម៉ាស៊ីនព្យាបាលទឹកប្រដាប់ជាតិអ៊ីដ្រូពោព
Ensuring your ម៉ាស៊ីនព្យាបាលទឹកប្រដាប់ជាតិអ៊ីដ្រូពោព meets all relevant safety standards requires a systematic approach. Follow these steps to verify compliance before making your investment:

- Request Documentation: Ask the manufacturer or supplier for copies of all certification documents, including CE marking, FDA clearance (if applicable), and ISO 13485 certification.
- Verify Certifications: Check the validity of certifications through official databases or by contacting certification bodies directly.
- Inspect Physical Markings: Examine the device for proper labeling, including CE mark, serial number, and manufacturer information.
- Review Technical Specifications: Ensure the machine meets specific requirements for temperature control, pressure regulation, and backflow prevention.
- Consult with Experts: When in doubt, seek advice from regulatory consultants or professional associations familiar with colon hydrotherapy equipment.
Warning: Be wary of suppliers who cannot provide proper certification documentation or who make claims about “certification equivalence” without supporting evidence. Using non-compliant equipment puts patients at risk and may invalidate your insurance coverage.
Regional Regulations for Colon Hydrotherapy Machines
Safety requirements for Colon Hydrotherapy Machines vary by region. Understanding these differences is essential when purchasing equipment, especially if you operate internationally or serve clients from different regions.
European Union
- CE marking mandatory
- EN 1717 backflow prevention
- Medical Device Regulation (MDR) compliance
- Requires clinical evaluation documentation
- Post-market surveillance system
United Kingdom
- UKCA marking (post-Brexit)
- MHRA registration required
- Water regulations approval scheme
- Professional association endorsements
- Follows similar standards to EU MDR
អាមេរិកខាងជើង
- FDA clearance in the United States
- Health Canada licensing
- UL/CSA electrical safety standards
- State/provincial regulations may apply
- Professional liability insurance requirements
Need Region-Specific Compliance Information?
Our international regulatory experts can help you navigate the specific requirements for your region.
Buyer’s Safety Checklist for Colon Hydrotherapy Machines
Use this comprehensive checklist when evaluating Colon Hydrotherapy Machines to ensure you’re investing in equipment that meets all essential safety standards.

Certification Verification
- CE marking (European markets)
- FDA clearance (US market)
- ISO 13485 quality management certification
- IEC 60601-1 electrical safety compliance
- EN 1717 backflow prevention compliance
- Local/regional certifications as applicable
Safety Features Assessment
- Automatic temperature control and shutdown
- Pressure regulation and relief systems
- Backflow prevention mechanisms
- Self-cleaning and disinfection capabilities
- Emergency stop function
- Disposable components availability
Documentation Requirements
- User manual with safety instructions
- Maintenance and cleaning protocols
- Certification documentation
- Technical specifications
- Warranty information
- Service and support details
Supplier Evaluation
- Manufacturer reputation and history
- Technical support availability
- Spare parts availability
- Training and implementation support
- Compliance with medical device regulations
- Post-purchase service commitments
Pro Tip: Request a demonstration of the machine’s safety features before purchase. Reputable suppliers will be happy to show how their equipment meets or exceeds safety standards and will provide comprehensive documentation to support their claims.
Frequently Asked Questions About ម៉ាស៊ីនព្យាបាលទឹកប្រដាប់ជាតិអ៊ីដ្រូពោព Safety

How often should Colon Hydrotherapy Machines be inspected for safety compliance?
Most regulatory bodies recommend annual safety inspections by qualified technicians. However, some regions may require more frequent inspections, especially for high-volume clinics. Daily operational checks should also be performed by operators to ensure all safety systems are functioning properly.
Can I use a Colon Hydrotherapy Machine certified in one region in a different country?
This depends on the specific regulations of each country. While some certifications (like CE marking) are recognized internationally, you’ll need to verify that the machine meets all local requirements. In many cases, additional certifications or modifications may be necessary to comply with local standards.
What are the most common safety issues with Colon Hydrotherapy Machines?
The most frequent safety concerns include inadequate backflow prevention (risking contamination), improper temperature control (risking patient burns or discomfort), and insufficient pressure regulation (potentially causing patient injury). These issues are typically addressed by proper certification and regular maintenance.
Are there different safety standards for clinic versus home-use Colon Hydrotherapy Machines?
Yes, professional clinic equipment typically must meet stricter standards than home-use devices. Many regions don’t permit home-use devices at all due to safety concerns. Professional equipment includes additional safety features and requires trained operation to minimize risks.
Ensuring Your ម៉ាស៊ីនព្យាបាលទឹកប្រដាប់ជាតិអ៊ីដ្រូពោព Meets Safety Standards
Investing in a ម៉ាស៊ីនព្យាបាលទឹកប្រដាប់ជាតិអ៊ីដ្រូពោព that meets all relevant safety standards is crucial for patient wellbeing, regulatory compliance, and the success of your practice. By understanding certification requirements, verifying compliance, and working with reputable suppliers, you can ensure your equipment provides safe and effective treatments.
Remember that safety standards continue to evolve as technology advances and regulatory frameworks develop. Staying informed about these changes and maintaining your equipment according to manufacturer specifications will help you provide the highest standard of care while protecting your practice.
Need Expert Guidance on ម៉ាស៊ីនព្យាបាលទឹកប្រដាប់ជាតិអ៊ីដ្រូពោព Safety?
Our team of specialists can help you navigate safety standards, verify certifications, and select equipment that meets all regulatory requirements for your region.
ទាក់ទងមកយើងខ្ញុំដោយ WhatsApp:

