Colon Hydrotherapy Device Manufacturer
Colon Hydrotherapy Machine Import Guide for US Buyers
October 16, 2025
Types of Colon Hydrotherapy Machines Available for Import









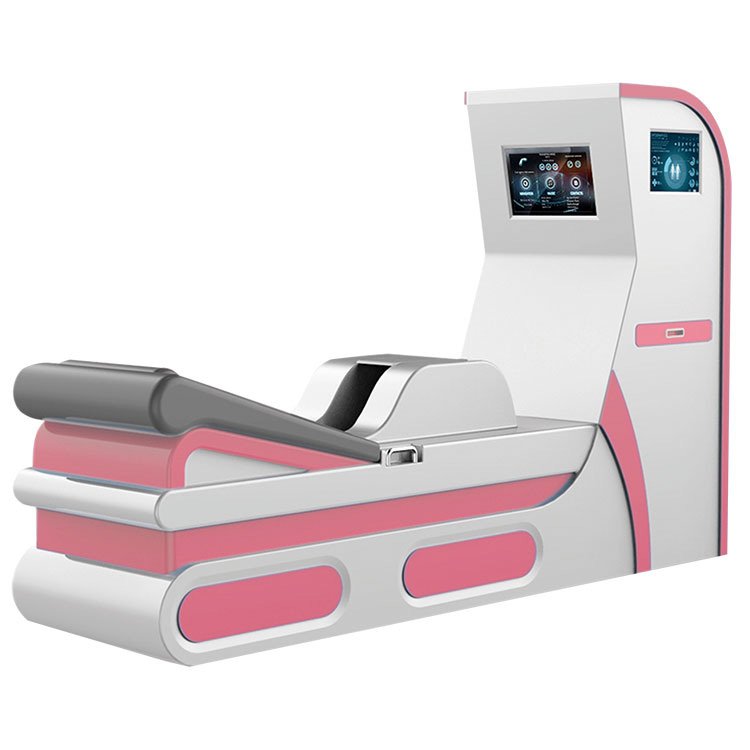

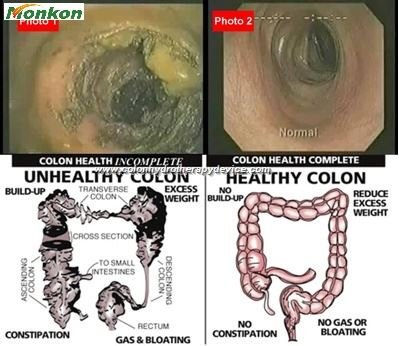
Understanding the different types of Colon Hydrotherapy Machines available is crucial for making an informed import decision. Each type offers specific features and requires different levels of regulatory compliance.
FDA-Approved Models
These Colon Hydrotherapy Machines have undergone rigorous testing and received FDA 510(k) clearance. They meet all US safety standards and can be legally marketed for medical use in clinics and hospitals.
- Fully compliant with US medical device regulations
- Typically higher cost but lower legal risk
- Often include comprehensive warranties and support
- Required for medical facilities billing insurance
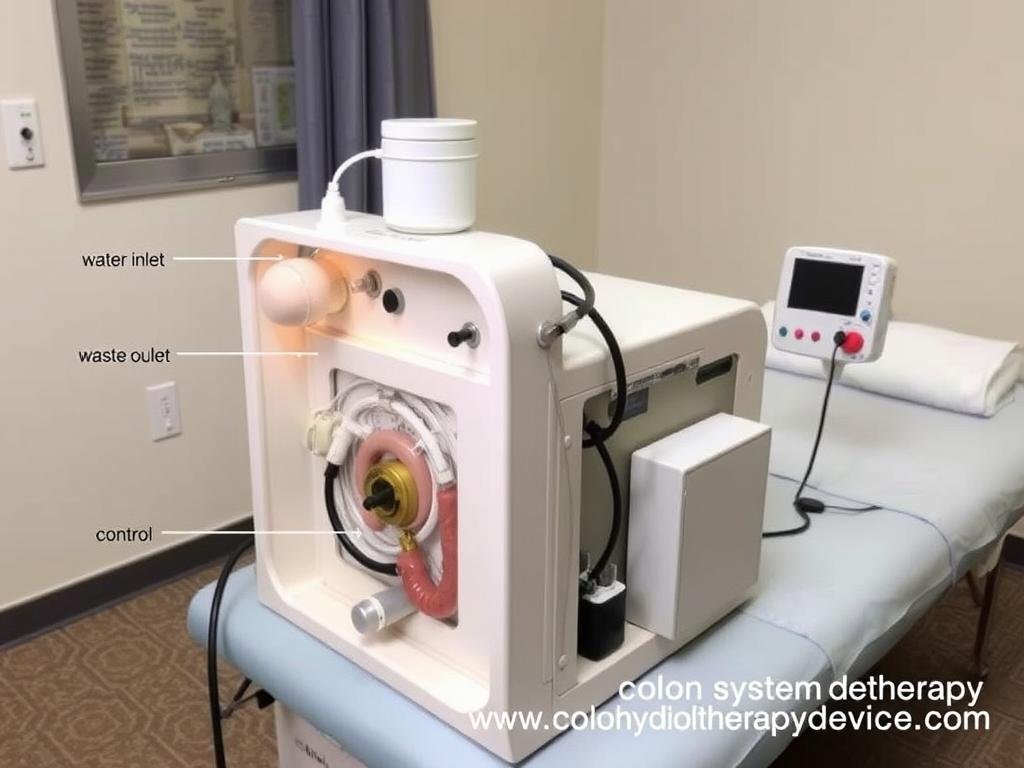
Open System Devices
Open system Colon Hydrotherapy Machines require a therapist to control water flow and temperature. These systems typically feature separate intake and waste disposal mechanisms and are commonly used in specialized hydrotherapy clinics.
- Therapist-operated for personalized treatments
- Typically require professional installation
- More complex regulatory requirements
- Higher level of operator training needed

Closed System Devices
Closed system Colon Hydrotherapy Machines use pre-packaged, disposable components for each session. These systems offer enhanced infection control and are often preferred in multi-practitioner settings.
- Enhanced infection control with disposable kits
- Simplified operation with pre-set parameters
- Lower maintenance requirements
- Often preferred for spa and wellness centers
Need Expert Guidance on Machine Selection?
Our specialists can help you determine which Colon Hydrotherapy Machine best suits your facility’s needs while ensuring FDA compliance.
US Import Regulations for Colon Hydrotherapy Machines
Importing Colon Hydrotherapy Machines to the United States involves navigating several regulatory bodies, with the FDA being the primary authority. Understanding these requirements is essential to avoid costly delays and potential legal issues.

FDA Requirements
The FDA classifies most Colon Hydrotherapy Machines as Class II medical devices, requiring:
- 510(k) premarket notification submission
- Registration of the manufacturing facility
- Listing of the device with the FDA
- Compliance with Quality System Regulation (QSR)
- Proper labeling according to FDA guidelines
For devices without FDA clearance, options include:
- Importing as “For Export Only” (not for US sale)
- Importing for research or educational purposes
- Applying for a Compassionate Use exemption
- Working with a US agent to obtain proper clearance
Important Note: Operating non-FDA cleared Colon Hydrotherapy Machines in a clinical setting may expose practitioners to significant legal liability and potential FDA enforcement actions.
Customs and Border Protection Requirements
In addition to FDA regulations, US Customs and Border Protection (CBP) oversees the physical importation process:
| Requirement | Description | Required Documents |
| Entry Documentation | Formal entry required for medical devices valued over $2,500 | CBP Form 3461, Commercial Invoice, Packing List |
| Tariff Classification | Proper HTS code assignment for Colon Hydrotherapy Machines | Product specifications, Technical documentation |
| FDA Prior Notice | Notification to FDA before arrival | FDA Form 2877, Device Listing Number |
| Import Bonds | Financial guarantee for duties and taxes | CBP Form 301, Surety provider information |
Required Certifications
Beyond FDA clearance, Colon Hydrotherapy Machines typically require additional certifications:

Electrical Safety
UL or ETL certification confirming the device meets US electrical safety standards.

Quality Management
ISO 13485 certification for the manufacturing facility demonstrates adherence to medical device quality standards.

Electromagnetic Compatibility
EMC certification ensures the device won’t interfere with other medical equipment.
Struggling with FDA Compliance?
Our team specializes in helping US buyers navigate the complex regulatory landscape for Colon Hydrotherapy Machines.
Cost Breakdown for Importing Colon Hydrotherapy Machines
Understanding the total cost of importing a Colon Hydrotherapy Machine involves more than just the purchase price. This comprehensive breakdown helps buyers plan their budget accurately.
| Cost Component | Typical Range (USD) | Notes |
| Base Equipment Cost | $3,000 – $15,000 | Varies significantly based on type, features, and certification status |
| International Shipping | $500 – $2,000 | Depends on origin country, shipping method, and machine size |
| Customs Duties | 7% – 10% of value | Based on HTS classification and country of origin |
| Customs Broker Fees | $200 – $500 | For handling import documentation and clearance |
| FDA Registration | $5,000 – $15,000 | If device requires new 510(k) submission |
| US Agent Fees | $1,000 – $3,000 annually | Required for foreign manufacturers |
| Installation & Training | $500 – $2,000 | Often higher for complex open systems |
| Warranty & Service Plan | $1,000 – $3,000 | Highly recommended for specialized equipment |
Cost-Saving Strategies
Recommended Approaches
- Import through established US distributors who handle FDA compliance
- Consider refurbished FDA-approved equipment from reputable sources
- Bundle orders with other clinics to reduce per-unit shipping costs
- Work with suppliers who offer comprehensive packages including installation
Approaches to Avoid
- Purchasing non-compliant equipment to save on initial costs
- Using general freight forwarders inexperienced with medical devices
- Skipping professional installation to reduce costs
- Opting out of service plans for specialized equipment
Get a Personalized Cost Estimate
Contact us for a detailed quote tailored to your specific Colon Hydrotherapy Machine requirements and location.
Selecting Reliable Colon Hydrotherapy Machine Suppliers
Choosing the right supplier is perhaps the most critical decision in the import process. A reputable supplier ensures product quality, regulatory compliance, and ongoing support.

Supplier Evaluation Criteria
Documentation to Request from Suppliers
Regulatory Documentation
- FDA 510(k) clearance letter
- FDA establishment registration
- Device listing confirmation
- Declaration of Conformity
- UL/ETL certification documents
Quality Assurance Documentation
- ISO 13485 certification
- Quality Management System manual
- Manufacturing process validation
- Test reports for safety and performance
- Material biocompatibility documentation
Commercial Documentation
- Detailed product specifications
- User and service manuals
- Warranty terms and conditions
- Parts availability guarantee
- Training program details
Warning: Be wary of suppliers who cannot provide proper FDA documentation or who suggest ways to bypass regulatory requirements. This can lead to serious legal consequences and potential equipment seizure by US authorities.
Need Help Vetting Suppliers?
Our team can help you evaluate potential Colon Hydrotherapy Machine suppliers and verify their regulatory compliance.
Step-by-Step Import Process for Colon Hydrotherapy Machines
Successfully importing a Colon Hydrotherapy Machine requires careful planning and execution. This step-by-step guide outlines the complete process from supplier selection to installation.
Pre-Import Phase
- Research and select FDA-compliant Colon Hydrotherapy Machine models
- Verify supplier credentials and regulatory documentation
- Request detailed quotations including shipping and warranty
- Confirm electrical compatibility with US standards (110V/60Hz)
- Review and negotiate payment terms and conditions
- Obtain written confirmation of FDA compliance status
Import Documentation Phase
- Engage a customs broker experienced with medical devices
- Prepare FDA Prior Notice submission
- Obtain commercial invoice and packing list from supplier
- Secure proper product classification (HTS code)
- Arrange for import bond through customs broker
- Prepare FDA Form 2877 for medical device import
Post-Import Phase
- Arrange for professional installation and setup
- Schedule staff training with supplier or authorized trainer
- Register warranty with manufacturer
- Establish maintenance schedule according to manufacturer guidelines
- Maintain records of all import and compliance documentation
- Set up protocol for ordering consumables and replacement parts
Common Import Challenges and Solutions
FDA Hold Challenges
FDA may place imported Colon Hydrotherapy Machines on hold for inspection if documentation is incomplete or raises concerns.
Solution: Work with suppliers who provide comprehensive FDA documentation packages and have experience with US imports. Consider using an FDA regulatory consultant for complex situations.
Customs Valuation Issues
Incorrect customs valuation can lead to delays and potential penalties.
Solution: Ensure all commercial documentation accurately reflects the true transaction value. Include all relevant costs such as tooling, engineering, and royalties in the declared value.
Shipping Damage
Sensitive medical equipment like Colon Hydrotherapy Machines can be damaged during international transport.
Solution: Request specialized medical equipment packaging, purchase shipping insurance, and document condition with photos before shipping. Perform thorough inspection upon arrival before signing delivery acceptance.
Installation Complications
Many facilities encounter unexpected installation challenges with water connections, drainage, or electrical requirements.
Solution: Request detailed installation specifications before purchase and have your facility assessed by a qualified technician. Budget for potential facility modifications.
Ready to Start Your Import Process?
Our team can guide you through each step of importing your Colon Hydrotherapy Machine to ensure a smooth process.
Get Expert Assistance with Your Colon Hydrotherapy Machine Import
Importing a Colon Hydrotherapy Machine doesn’t have to be complicated. Our team of experts specializes in helping US buyers navigate the entire process from selection to installation.

“Working with an experienced supplier made all the difference in our import process. Their guidance on FDA requirements saved us significant time and prevented potential compliance issues.”
How We Can Help
- FDA compliance guidance and documentation
- Supplier verification and quality assurance
- Detailed cost estimates and budget planning
- Customs clearance coordination
- Installation and training arrangements
- Ongoing technical support and parts sourcing
+86 13510907401WhatsApp Available
Conclusion
Importing a Colon Hydrotherapy Machine to the United States requires careful attention to regulatory requirements, supplier selection, and logistical planning. By following the guidelines in this comprehensive guide, US buyers can navigate the import process successfully while ensuring compliance with all applicable regulations.
Remember that working with experienced suppliers and consultants can significantly streamline the process and help avoid costly mistakes. Whether you’re a medical clinic, wellness center, or spa looking to add colon hydrotherapy services, proper planning and expert guidance are key to a successful import experience.
Ready to Import Your Colon Hydrotherapy Machine?
Contact Lucy today for a free consultation on FDA-approved Colon Hydrotherapy Machines and personalized import guidance.
Contact us By WhatsApp:

