Colon Hydrotherapy Device Manufacturer
FDA-Approved Colon Hydrotherapy Machine: What’s Available on the Market?
October 20, 2025
















Understanding FDA Requirements for Colon Hydrotherapy Machines
The FDA classifies colon hydrotherapy machines as Class II medical devices, requiring specific safety standards and regulatory compliance. These requirements ensure that the equipment used in medical facilities and wellness centers meets strict quality control measures for patient safety.
Key FDA Compliance Factors
- 510(k) premarket notification submission
- Quality System Regulation (QSR) adherence
- Medical Device Reporting (MDR) compliance
- Proper labeling and instructions for use
- Risk assessment documentation

Ensure Your Facility Meets Regulatory Standards
MAIKONG provides fully documented FDA-compliant colon hydrotherapy machines with all necessary certification for immediate implementation in your facility.
Benefits of FDA-Approved Colon Hydrotherapy Equipment
Investing in FDA-approved colon hydrotherapy equipment offers significant advantages for both practitioners and patients. These benefits extend beyond mere regulatory compliance to impact your facility’s reputation, operational efficiency, and patient outcomes.
Enhanced Credibility
FDA approval signals to patients and healthcare partners that your facility maintains the highest standards of care and equipment quality.

Superior Safety Profile
FDA-approved devices incorporate mandatory safety features including temperature controls, pressure regulation, and contamination prevention systems.
Insurance Compatibility
Many insurance providers require FDA-approved equipment for treatment coverage, potentially increasing your client base and revenue streams.
Upgrade Your Practice with FDA-Approved Technology
MAIKONG’s colon hydrotherapy machines combine regulatory compliance with advanced features for optimal treatment outcomes.
MAIKONG Premium Colon Hydrotherapy Machines
MAIKONG has established itself as a leading manufacturer of FDA-approved colon hydrotherapy equipment, offering a range of models designed to meet different facility requirements while maintaining strict compliance with regulatory standards.

Featured Model: MAIKONG MK-9000 Colon Hydrotherapy System
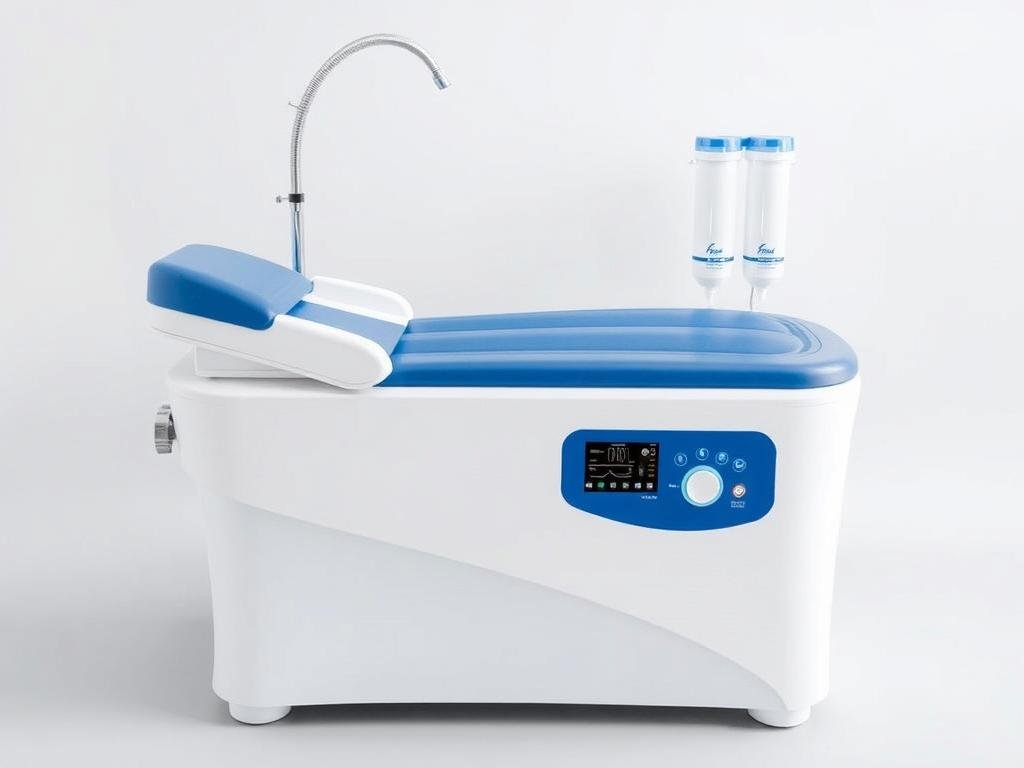
Key Specifications
| Feature | Specification |
| Water Pressure Range | 860-1060 hPa (adjustable) |
| Water Temperature | 10-40°C (±2°C precision) |
| Flow Rate | Up to 2L/min (controlled) |
| Filtration System | Multi-stage with 1μm accuracy |
| Display | Dual LCD touchscreens |
| FDA Compliance | Full Class II certification |
Advantages
- Fully automated pressure and temperature control
- Disposable kit system for maximum hygiene
- Integrated UV water sterilization
- User-friendly interface for practitioners
- Comprehensive safety monitoring systems
- Remote technical support capability
Considerations
- Premium pricing compared to non-FDA models
- Requires dedicated water connection
- Professional installation recommended
- Annual maintenance schedule required
Comparing FDA-Approved Colon Hydrotherapy Machines
When evaluating FDA-approved options, it’s important to compare key features that impact treatment efficacy, safety, and operational efficiency. The table below compares MAIKONG’s flagship model against other available FDA-approved systems.
| Feature | MAIKONG MK-9000 | Competitor A | Competitor B |
| FDA Approval Status | Full Class II Certification | Partial Compliance | Full Class II Certification |
| Water Filtration | 4-Stage with UV | 3-Stage | 3-Stage with UV |
| Temperature Control | ±1°C Precision | ±2°C Precision | ±2°C Precision |
| Disposable Components | Full Kit System | Partial Kit | Full Kit System |
| Warranty | 3 Years | 1 Year | 2 Years |
| Technical Support | 24/7 Remote + On-site | Business Hours Only | Extended Hours |

Safety Features in Modern Colon Hydrotherapy Equipment
FDA-approved colon hydrotherapy machines incorporate multiple safety systems to protect patients and ensure reliable operation. MAIKONG’s commitment to safety goes beyond minimum requirements to provide comprehensive protection.

Water Purification
Multi-stage filtration systems with UV sterilization ensure water purity throughout the treatment process, eliminating potential contaminants.

Pressure Regulation
Automated pressure monitoring with instant cutoff prevents excessive pressure that could cause patient discomfort or injury during treatment.
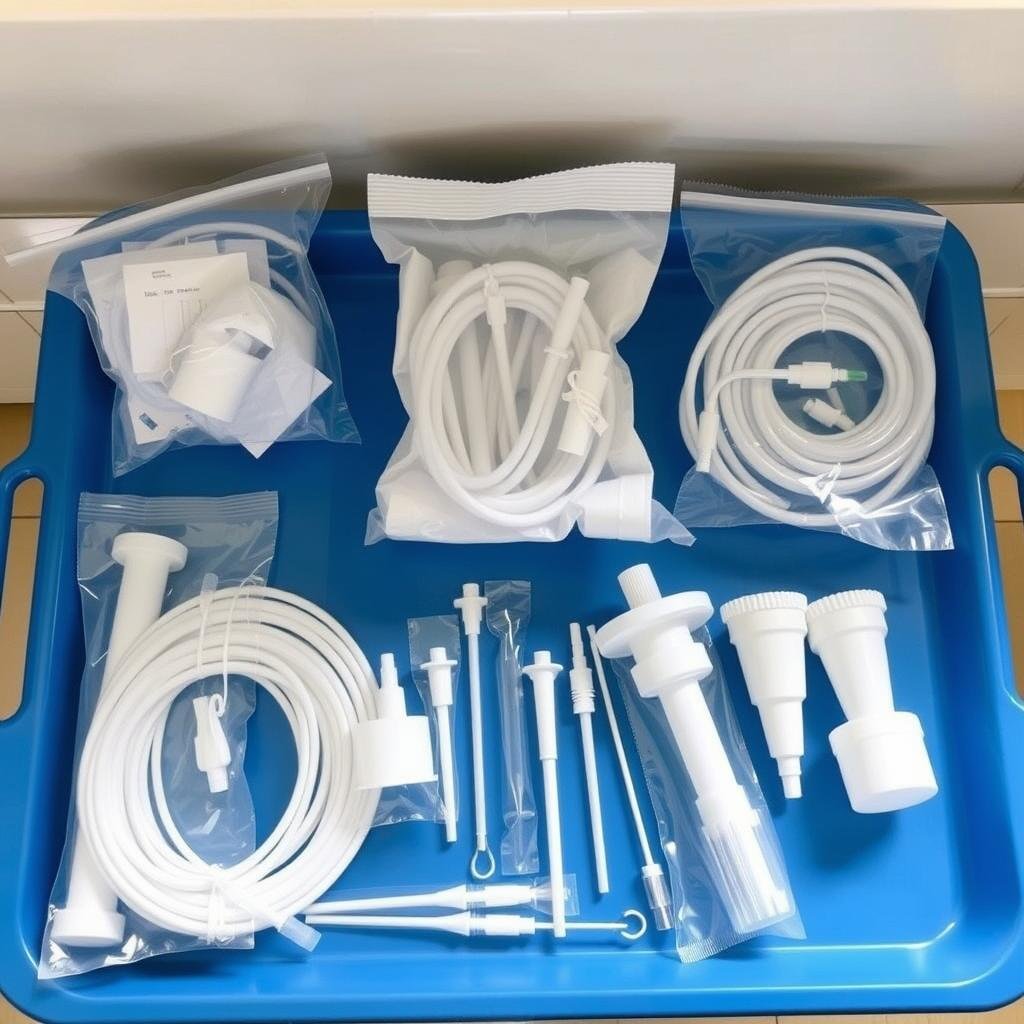
Cross-Contamination Prevention
Single-use disposable components eliminate the risk of cross-contamination between patients, ensuring the highest hygiene standards.
“MAIKONG’s safety systems exceed FDA requirements, providing multiple redundant protections that give practitioners confidence and patients peace of mind during treatment.”
Implementing Colon Hydrotherapy Machines in Your Facility
Successfully integrating an FDA-approved colon hydrotherapy machine into your practice requires careful planning. MAIKONG provides comprehensive support throughout this process to ensure a smooth transition.
Implementation Process
- Facility AssessmentOur specialists evaluate your space requirements, water connections, and electrical capacity to ensure compatibility.
- Regulatory DocumentationWe provide all necessary FDA compliance documentation for your facility records and insurance requirements.
- Professional InstallationCertified technicians handle the complete installation and initial calibration of your equipment.
- Staff TrainingComprehensive training ensures your team can operate the equipment safely and effectively.
- Ongoing SupportTechnical assistance and maintenance programs keep your equipment operating at peak performance.


Space Requirements: MAIKONG colon hydrotherapy machines typically require a dedicated treatment room of at least 120 sq ft (11 sq m) with access to water supply, drainage, and appropriate electrical outlets. Our planning team can help optimize your existing space.
Frequently Asked Questions About FDA-Approved Colon Hydrotherapy Machines
What makes a colon hydrotherapy machine FDA-approved?
FDA approval requires manufacturers to demonstrate that their colon hydrotherapy equipment meets specific safety and efficacy standards through the 510(k) premarket notification process. This includes rigorous testing of all components, comprehensive documentation of safety systems, and adherence to Quality System Regulations (QSR).
How often does FDA-approved equipment need maintenance?
MAIKONG recommends quarterly preventive maintenance checks and an annual comprehensive service to ensure optimal performance and continued compliance with FDA standards. Our service agreements include these scheduled maintenance visits to protect your investment.
Can non-medical facilities purchase FDA-approved colon hydrotherapy machines?
Yes, wellness centers and alternative health facilities can purchase FDA-approved equipment. However, operators should verify local regulations regarding who can perform colon hydrotherapy treatments, as this varies by state and locality.
What is the typical return on investment for a premium colon hydrotherapy machine?
Most facilities report a complete return on investment within 12-18 months, depending on treatment volume and pricing. FDA-approved equipment typically commands higher treatment fees and attracts more insurance-covered patients, accelerating ROI compared to non-approved alternatives.
Choose MAIKONG for FDA-Approved Colon Hydrotherapy Solutions
Investing in an FDA-approved colon hydrotherapy machine represents a commitment to patient safety, regulatory compliance, and treatment quality. MAIKONG’s premium equipment combines advanced technology with comprehensive support to provide the ideal solution for medical facilities and wellness centers.

Ready to Upgrade Your Facility with FDA-Approved Technology?
Contact our specialists today to discuss your specific requirements and discover how MAIKONG’s colon hydrotherapy machines can enhance your practice.
Contact on WhatsAppPhone/WhatsApp: +86 13510907401
Email UsEmail: Lucy@colonhydrotherapydevice.com
Website: colonhydrotherapydevice.com
Contact us By WhatsApp:

